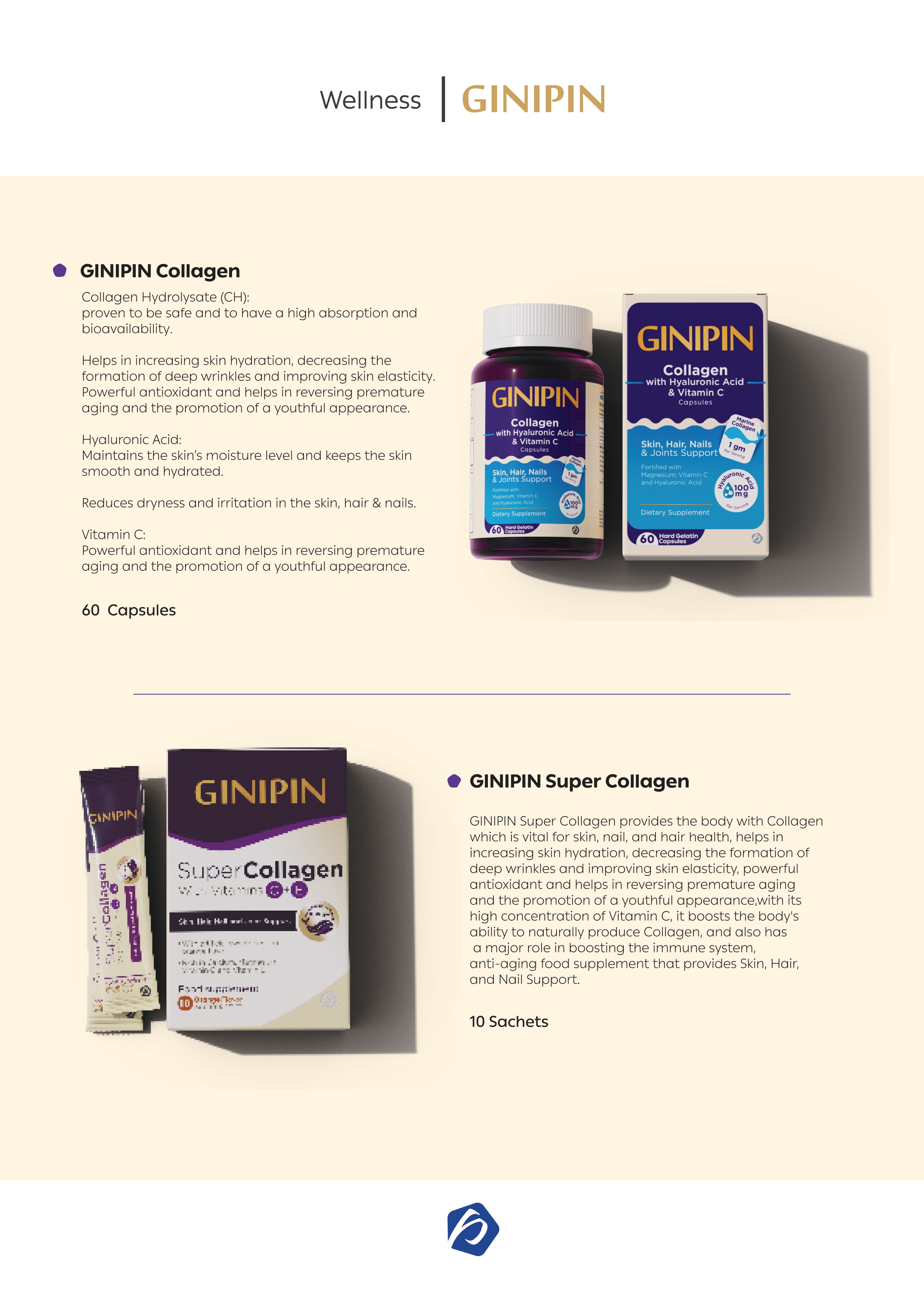
Imported Cosmetic Products Requirements
Already registered with an old agent (if there is no hard copy of registration license):
- Hard copy composition list.
- Hard copy of pack artwork traded in the country of origin with full data.
- Termination letter.
- Authorization letter from the owner company of the products to the importer in Egypt showing that the importer company has the legal right to register the products (names of the products) in Egypt. This letter must be legalized by the Chamber of Commerce and the Egyptian Embassy or the Egyptian consulate in the country of origin.
Authorization letter form: xxx company (full data) authorizes Parkville (full data) to distribute and register our products (cosmetics, medical devices, etc.) in Egypt.
New Registration:
- Free sale certificate from the country of origin, legalized by the Chamber of Commerce, Chamber of Industry and Commerce, Ministry of Health, Ministry of Industry, or notifying body, and legalized by the Egyptian Embassy in the country of origin. This certificate must include the names of products to be registered, as well as the name of the manufacturer, confirming that the products are freely sold in the country of origin with the same name and composition.
- Shelf-Life Statement stamped and legalized by the Chamber of Commerce or notifying body, and legalized by the Egyptian Embassy in the country of origin.
- Composition list to be sent by email.
- Pack artwork traded in the country of origin with full data (PDF) to be sent by email.
- Authorization letter from the owner company of the products to the importer in Egypt showing that the importer company has the legal right to register the products (names of the products) in Egypt. This letter must be legalized by the Chamber of Commerce and the Egyptian Embassy or the Egyptian consulate in the country of origin.
Authorization letter form: xxx company (full data) authorizes Parkville (full data) to distribute and register our products (cosmetics, medical devices, etc.) in Egypt.
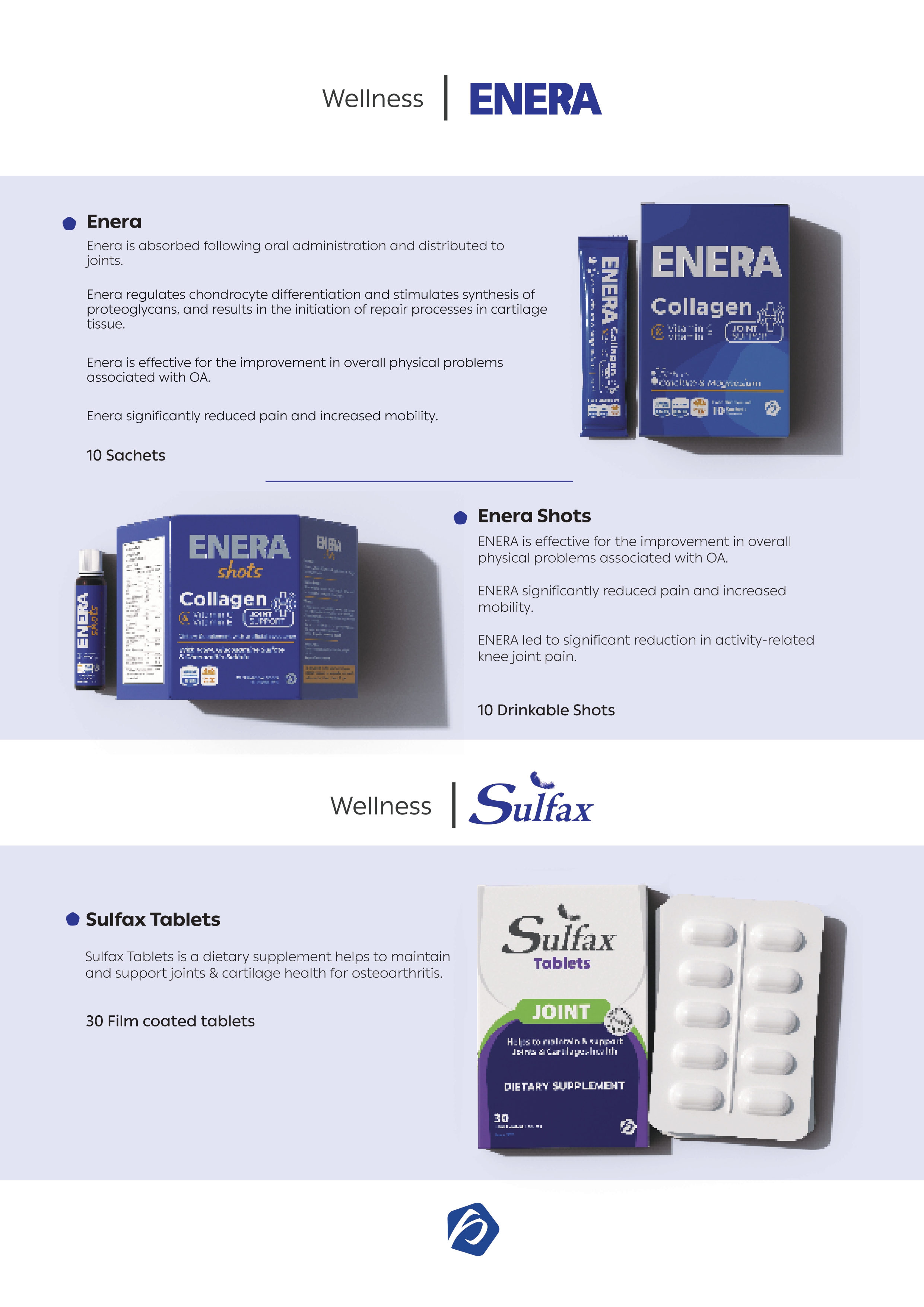
Imported Medical Device Requirements
- CE Certificate of final product including ingredients, accessories, and classification details.
- TSE-Free directive CE certificate for products with animal-origin ingredients.
- ISO 13485:2003 certification issued by an accredited body.
- Signed and stamped Declaration of Conformity, including product classification, quality assurance details, and indication of use.
- Technical File signed and stamped on producer letterhead.
- Certificates of analysis for physical, chemical, and biological properties.
- Sterilization and packaging materials certificates.
- Inner and outer label descriptions with samples of the master label.
- Medical device full manual.
- Shelf life and storage conditions.
About Us